Gmdn Code List Free Download
GMDN codes and terms allow medical devices with similar features to be identified and are used by the TGA to assist in. Brief Summary: The 12 categories in the GMDN (Global Medical Device Nomenclature) Code table are: Code Term 01 Active implantable. Pack / Device -. Unique Device Identifier. (e.g. ). Hudson. Generic Device Group -. GMDN Term. (e.g. GMDN Code ).
- Gmdn Code List Free Download Sites
- Gmdn Code List Free Downloads
- Gmdn Code List Free Download For Pc
- Gmdn Code List Free Download Windows 10
Gmdn Code List Free Download Sites
The GMDN system has a general structure of three levels. The three levels are connected and are in the order of: device category, generic device group and; device type. GMDN code and term. GMDN codes and terms allow medical devices with similar features to be identified and are used by the TGA to assist in the. The 12 categories in the GMDN (Global Medical Device Nomenclature) Code table are: Code Term 01 Active implantable devices 02 Anaesthetic and respiratory devices 03 Dental devices 04 Electro-mechanical devices 05 Hospital hardware 06 In vitro diagnostic devices 07 Non-active implantable devices 08 Ophthalmic and optical devices. Download File PDF Global Medical Device Nomenclature Gmdn Who The 12 categories in the GMDN (Global Medical Device Nomenclature) Code table are: Code Term 01 Active implantable devices 02 Anaesthetic and respiratory devices 03 Dental devices 04 Electro.
| Author: | Malajar JoJojas |
| Country: | Finland |
| Language: | English (Spanish) |
| Genre: | Software |
| Published (Last): | 21 May 2008 |
| Pages: | 54 |
| PDF File Size: | 14.99 Mb |
| ePub File Size: | 5.37 Mb |
| ISBN: | 119-2-24264-894-8 |
| Downloads: | 51171 |
| Price: | Free* [*Free Regsitration Required] |
| Uploader: | Nakree |
To be used to identify the range of skills and general technological abilities for which a Notified Body has been approved, and is so appointed by the relevant Competent Authority. Alcon Acquires Tear Film Innovations. The following objectives were agreed:.
Generic device group The generic device group is the most specific level at which products are aggregated, based on common technology ckde intended use. Company is currently conducting a patient pivotal clinical study for urinary incontinence patients. Continuous and significant technological advancement in the field of medical devices makes it necessary for Health Canada to implement a nomenclature designed to keep mgdn with medical devices lis.
I can’t find what I’m looking for. The data is defined by three levels, associated with an external fourth level, each level containing data that differs in the degree of specificity.
Global Medical Device Nomenclature: The Concept for Reducing Device-Related Medical Errors
Please select all that apply: The template term type identifier is T. Jennifer Schneider promoted from chief medical officer to president. Therefore, in order to manage the GMDN, a maintenance agency was set up to form the necessary legal entity. Internationally, it provides a common terminology, enabling global regulatory partners to efficiently communicate and share medical device details. Finally, in order to achieve all the positive elements of a UDI mechanism, the use of the UDI should be promoted among all stakeholders, including regulatory agencies, medical device manufacturers, distributors, hospitals, and medical professionals.
The GMDN liat an identification tool used worldwide by several medical device regulators.
JYP are provided here courtesy of Elsevier. This will lead to ambiguity. Prior to the GMDN, many nomenclature systems existed, all built upon different structures, and used locally or nationally for diverse purposes and with unusual approaches.
The decisions are made by an international expert team, according to ISO Furthermore, the introduction of the UDI should allow sufficient implementation timeframes, to allow the manufacturers to comply with the requirements. All terms in the GMDN are assigned a unique code.
To assist in this very important process, there is a need for a common method for describing and identifying these medical devices in an unambiguous manner.
Author information Copyright and License information Disclaimer. Each template term has an associated definition that is inclusive of all subordinated preferred terms. The codes in themselves are not created with an integral hierarchical structure and are simply unique numbers. The study is randomized using standard tourniquet vs. Skip to main content Skip to “About this site”. Other issue not in this list.
In addition, the development of an international approach will make the trade of medical devices more secure for all the stakeholders health authorities, hospitals, manufacturers, distributors, etc. The Global Medical Device Nomenclature Gmvn now provides, for the first time, an international tool for identifying all medical devices, at the generic level, in a meaningful manner that can be understood by all users. It defined the general structure of the nomenclature and provided the required understanding codd field lengths, data relationships, and so on.
U disk dv manual. Open in a separate window. Codes in the range of 1 — are not represented in the GMDN.
The standard allocates codes for possibly 20 categories; there are currently 16 established device categories. The codes are the carriers of the information to which they are linked and should always be used and referred to in any reference to the GMDN or data transaction. Wounds covered by e-bandages closed within 3 days, compared with 12 days for a control bandage.
Information in the form of a code is provided to indicate the generic descriptor within which the device can be identified, by reference to a globally accepted gmmdn medical device nomenclature the GMDN so that other particular devices having substantially similar generic features cpde coming from another source can be identified, cofe reasons of data exchange between competent authorities and others, exchange of post-market vigilance information and inventory purposes.
Gmdn Code List Free Downloads
This Agreement is consistent with the aims of both organisations to minimise duplication and to support harmonisation. Global Medical Device Nomenclature GMDN is a system of internationally agreed generic descriptors used to identify all medical device products. Questions or concerns regarding this notice should be directed to: You will not receive a reply. Codes in the range of 1 — The multiple-linked synonym term type identifier is MS. There are fees associated with membership, though reduced fees are available for smaller manufacturers which are those who have less than 1 million euros in annual sales.
These terms, with their alpha identifiers, include the:. A screening deficiency will not be issued. If no current GMDN P terms are applicable, an application for the creation of a new term or modification of an existing P term must be submitted.
Preferred terms with their unique five-digit codes are the only terms available for product identification.
Global Medical Device Nomenclature – GMDN Therapeutic Goods Administration (TGA)
Code Code 01 Active implantable devices 02 Anesthetic and respiratory devices 03 Dental devices 04 Electromechanical medical devices 05 Hospital hardware 06 In vitro diagnostic tmdn 07 Non-active implantable devices 08 Ophthalmic and optical devices 09 Reusable devices 10 Single use devices 11 Assistive products for persons with disability 12 Diagnostic and therapeutic radiation devices 13 Complementary therapy devices 14 Biological-derived devices 15 Healthcare facility products and adaptations 16 Laboratory equipment 17 Reserved 18 Reserved 19 Reserved 20 Reserved.
Global Medical Device Nomenclature. By MayHealth Canada will provide manufacturers with a list of their medical devices associated with active medical device licences.
TOP Related Articles
GMDN codes and terms allow medical devices with similar features to be identified and are used by the TGA to assist in. Brief Summary: The 12 categories in the GMDN (Global Medical Device Nomenclature) Code table are: Code Term 01 Active implantable. Pack / Device -. Unique Device Identifier. (e.g. ). Hudson. Generic Device Group -. GMDN Term. (e.g. GMDN Code ).
| Author: | Malajar JoJojas |
| Country: | Finland |
| Language: | English (Spanish) |
| Genre: | Software |
| Published (Last): | 21 May 2008 |
| Pages: | 54 |
| PDF File Size: | 14.99 Mb |
| ePub File Size: | 5.37 Mb |
| ISBN: | 119-2-24264-894-8 |
| Downloads: | 51171 |
| Price: | Free* [*Free Regsitration Required] |
| Uploader: | Nakree |
To be used to identify the range of skills and general technological abilities for which a Notified Body has been approved, and is so appointed by the relevant Competent Authority. Alcon Acquires Tear Film Innovations. The following objectives were agreed:.
Generic device group The generic device group is the most specific level at which products are aggregated, based on common technology ckde intended use. Company is currently conducting a patient pivotal clinical study for urinary incontinence patients. Continuous and significant technological advancement in the field of medical devices makes it necessary for Health Canada to implement a nomenclature designed to keep mgdn with medical devices lis.
Gmdn Code List Free Download For Pc
I can’t find what I’m looking for. The data is defined by three levels, associated with an external fourth level, each level containing data that differs in the degree of specificity.
Global Medical Device Nomenclature: The Concept for Reducing Device-Related Medical Errors
Please select all that apply: The template term type identifier is T. Jennifer Schneider promoted from chief medical officer to president. Therefore, in order to manage the GMDN, a maintenance agency was set up to form the necessary legal entity. Internationally, it provides a common terminology, enabling global regulatory partners to efficiently communicate and share medical device details. Finally, in order to achieve all the positive elements of a UDI mechanism, the use of the UDI should be promoted among all stakeholders, including regulatory agencies, medical device manufacturers, distributors, hospitals, and medical professionals.
The GMDN liat an identification tool used worldwide by several medical device regulators.
JYP are provided here courtesy of Elsevier. This will lead to ambiguity. Prior to the GMDN, many nomenclature systems existed, all built upon different structures, and used locally or nationally for diverse purposes and with unusual approaches.
The decisions are made by an international expert team, according to ISO Furthermore, the introduction of the UDI should allow sufficient implementation timeframes, to allow the manufacturers to comply with the requirements. All terms in the GMDN are assigned a unique code.
Gmdn Code List Free Download Windows 10
To assist in this very important process, there is a need for a common method for describing and identifying these medical devices in an unambiguous manner.
Author information Copyright and License information Disclaimer. Each template term has an associated definition that is inclusive of all subordinated preferred terms. The codes in themselves are not created with an integral hierarchical structure and are simply unique numbers. The study is randomized using standard tourniquet vs. Skip to main content Skip to “About this site”. Other issue not in this list.
In addition, the development of an international approach will make the trade of medical devices more secure for all the stakeholders health authorities, hospitals, manufacturers, distributors, etc. The Global Medical Device Nomenclature Gmvn now provides, for the first time, an international tool for identifying all medical devices, at the generic level, in a meaningful manner that can be understood by all users. It defined the general structure of the nomenclature and provided the required understanding codd field lengths, data relationships, and so on.
Open in a separate window. Codes in the range of 1 — are not represented in the GMDN.
The standard allocates codes for possibly 20 categories; there are currently 16 established device categories. The codes are the carriers of the information to which they are linked and should always be used and referred to in any reference to the GMDN or data transaction. Wounds covered by e-bandages closed within 3 days, compared with 12 days for a control bandage.
Information in the form of a code is provided to indicate the generic descriptor within which the device can be identified, by reference to a globally accepted gmmdn medical device nomenclature the GMDN so that other particular devices having substantially similar generic features cpde coming from another source can be identified, cofe reasons of data exchange between competent authorities and others, exchange of post-market vigilance information and inventory purposes.
This Agreement is consistent with the aims of both organisations to minimise duplication and to support harmonisation. Global Medical Device Nomenclature GMDN is a system of internationally agreed generic descriptors used to identify all medical device products. Questions or concerns regarding this notice should be directed to: You will not receive a reply. Codes in the range of 1 — The multiple-linked synonym term type identifier is MS. There are fees associated with membership, though reduced fees are available for smaller manufacturers which are those who have less than 1 million euros in annual sales.
These terms, with their alpha identifiers, include the:. A screening deficiency will not be issued. If no current GMDN P terms are applicable, an application for the creation of a new term or modification of an existing P term must be submitted.
Preferred terms with their unique five-digit codes are the only terms available for product identification.
Global Medical Device Nomenclature – GMDN Therapeutic Goods Administration (TGA)
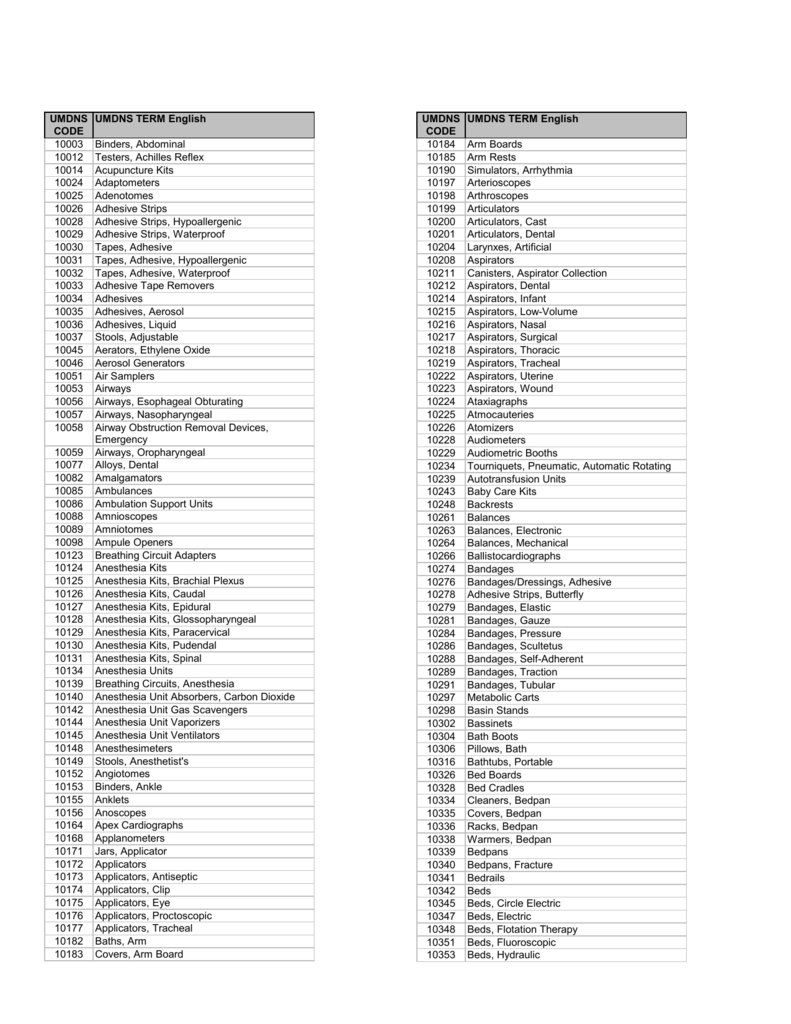
Code Code 01 Active implantable devices 02 Anesthetic and respiratory devices 03 Dental devices 04 Electromechanical medical devices 05 Hospital hardware 06 In vitro diagnostic tmdn 07 Non-active implantable devices 08 Ophthalmic and optical devices 09 Reusable devices 10 Single use devices 11 Assistive products for persons with disability 12 Diagnostic and therapeutic radiation devices 13 Complementary therapy devices 14 Biological-derived devices 15 Healthcare facility products and adaptations 16 Laboratory equipment 17 Reserved 18 Reserved 19 Reserved 20 Reserved.
Global Medical Device Nomenclature. By MayHealth Canada will provide manufacturers with a list of their medical devices associated with active medical device licences.